Bydureon Bcise Availability - The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from. Generic drug availability, manufacturer information, and patent status on bydureon bcise Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Drug manufacturer astrazeneca (az) recently notified the u.s. Food and drug administration (fda) that it discontinued. Reason for the shortage astrazeneca has discontinued bydureon bcise. Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Astrazeneca has discontinued bydureon pens.
Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Reason for the shortage astrazeneca has discontinued bydureon bcise. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from. Astrazeneca has discontinued bydureon pens. Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Generic drug availability, manufacturer information, and patent status on bydureon bcise Food and drug administration (fda) that it discontinued. Drug manufacturer astrazeneca (az) recently notified the u.s.
Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Drug manufacturer astrazeneca (az) recently notified the u.s. Generic drug availability, manufacturer information, and patent status on bydureon bcise Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Astrazeneca has discontinued bydureon pens. Reason for the shortage astrazeneca has discontinued bydureon bcise. Food and drug administration (fda) that it discontinued. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from.
US FDA approves new easytouse, onceweekly Bydureon BCise injectable
Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply.
Bydureon® BCise™ for the Treatment of Type2 Diabetes Clinical Trials
Drug manufacturer astrazeneca (az) recently notified the u.s. Food and drug administration (fda) that it discontinued. Generic drug availability, manufacturer information, and patent status on bydureon bcise Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when.
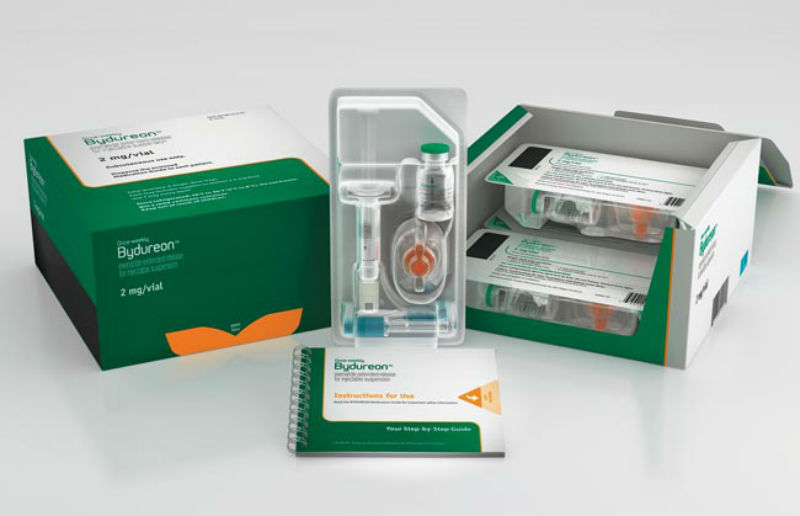
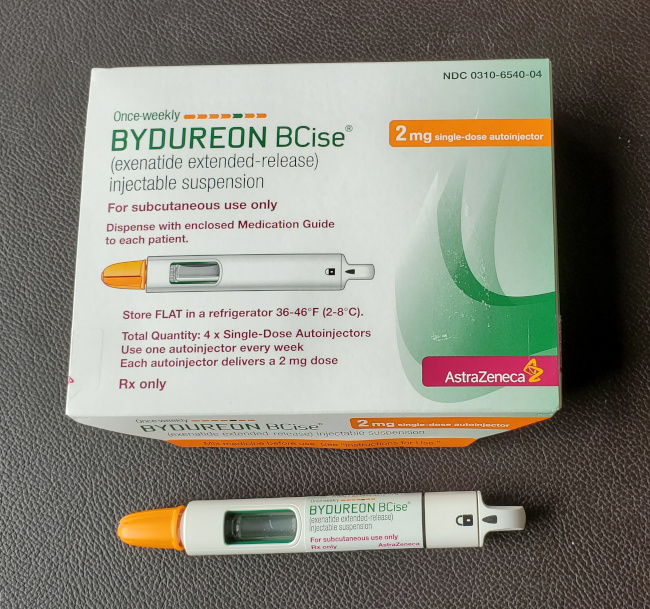
Bydureon Bcise Injection, 2mg, Packaging Size 4 X Single Dose
Astrazeneca has discontinued bydureon pens. The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from. Drug manufacturer astrazeneca (az) recently notified the u.s. Food and drug administration (fda).
Bydureon — Jack Dolci
Generic drug availability, manufacturer information, and patent status on bydureon bcise Reason for the shortage astrazeneca has discontinued bydureon bcise. Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. The list below contains full prescribing information for all of our medicines and a range of websites.
Drugs and Therapies in Trials for Parkinson's Disease
Astrazeneca has discontinued bydureon pens. Drug manufacturer astrazeneca (az) recently notified the u.s. Food and drug administration (fda) that it discontinued. Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from.
BYDUREON BCise Healthgrades (exenatide injection, suspension
Generic drug availability, manufacturer information, and patent status on bydureon bcise Reason for the shortage astrazeneca has discontinued bydureon bcise. Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Per astrazeneca,.
Product Images Bydureon Bcise Photos Packaging, Labels & Appearance
Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Food and drug administration (fda) that it discontinued. The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Reason for the shortage astrazeneca has discontinued.
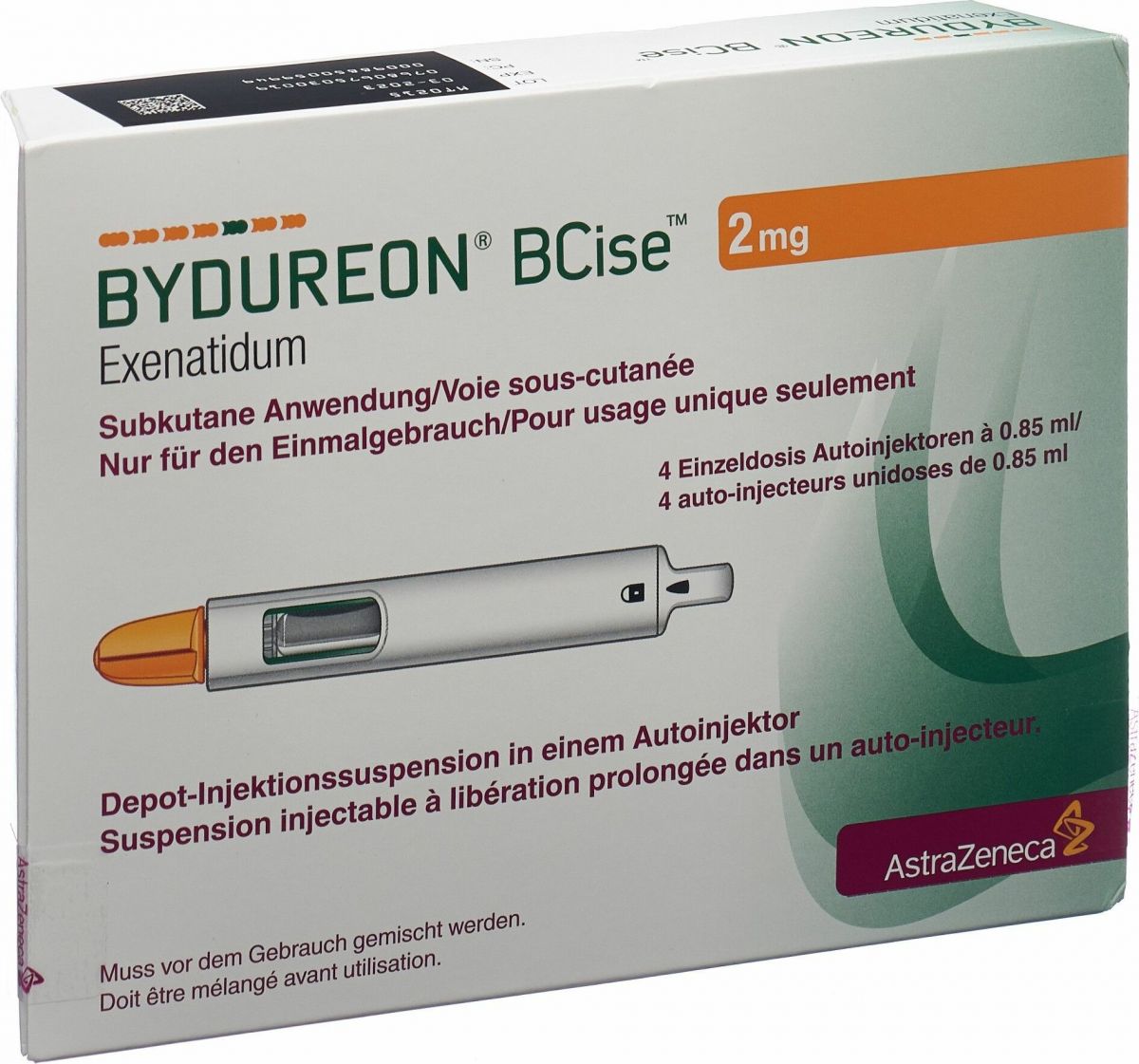
Bydureon Bcise Depot 2mg Autoinjektor 4 Stück in der Adler Apotheke
Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Reason for the shortage astrazeneca has discontinued bydureon bcise. Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Generic drug availability, manufacturer information, and patent status on bydureon bcise Per.
Bydureon Bcise Exenatide Extendedrelease Injectable Etsy
Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Per astrazeneca, there is adequate supply of bydureon bcise and there are no anticipated supply issues when patients switch from. The list below contains full prescribing information for all of our medicines and a range of websites.
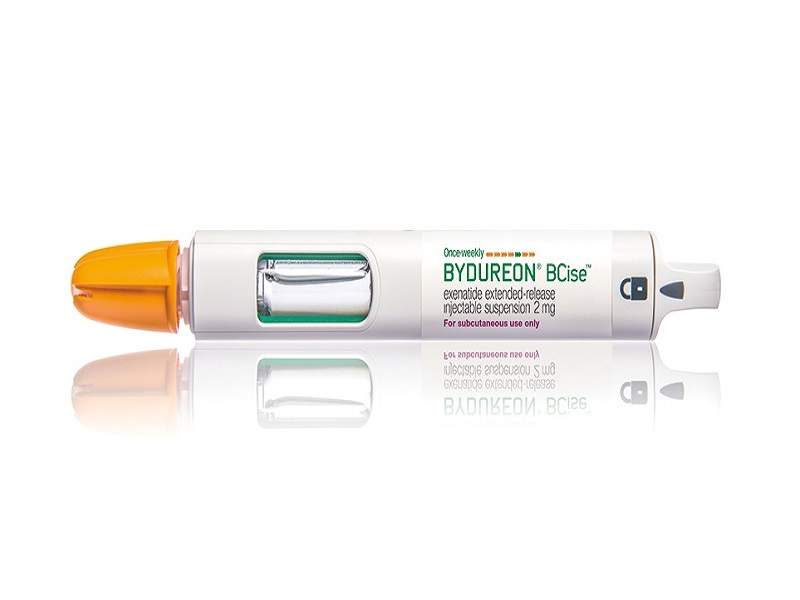
Bydureon® Bcise™ Injectable Medicine Now Available In The Us For Patients
Generic drug availability, manufacturer information, and patent status on bydureon bcise The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with. Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Astrazeneca has discontinued bydureon.
Per Astrazeneca, There Is Adequate Supply Of Bydureon Bcise And There Are No Anticipated Supply Issues When Patients Switch From.
Order bydureon bcise® exenatide microspheres 2 mg injection 4 pens 4 pens by astrazeneca pharmaceuticals 00310654004 Food and drug administration (fda) that it discontinued. Counsel patients regarding the potential risk for mtc with the use of bydureon bcise and inform them of symptoms of thyroid tumors (e.g.,. Drug manufacturer astrazeneca (az) recently notified the u.s.
Astrazeneca Has Discontinued Bydureon Pens.
Generic drug availability, manufacturer information, and patent status on bydureon bcise Reason for the shortage astrazeneca has discontinued bydureon bcise. The list below contains full prescribing information for all of our medicines and a range of websites dedicated to providing you with.