Commercially Available Starting Material - This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. Port a regulatory starting material proposal to an agency. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. And the better packages are ones that are supported by data.the reason this is important. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for.
This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. Port a regulatory starting material proposal to an agency. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for. And the better packages are ones that are supported by data.the reason this is important.
A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. And the better packages are ones that are supported by data.the reason this is important. Port a regulatory starting material proposal to an agency. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and.
Figure 3 from Identification of Commercially Available Oligonucleotide
Port a regulatory starting material proposal to an agency. And the better packages are ones that are supported by data.the reason this is important. This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided.
(PDF) Straightforward synthesis of aliphatic polydithiocarbonates from
This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. And the better packages are ones that are supported by data.the reason this is important. Port a regulatory.
Automation of Manufacturing Process ppt download
A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. And the better packages are ones that are supported by data.the reason this is important. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is.
Figure 5 from Identification of Commercially Available Oligonucleotide
And the better packages are ones that are supported by data.the reason this is important. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as.
In Situ Generation of a Regio and Diastereoselective
Port a regulatory starting material proposal to an agency. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. And the better packages are ones that are supported by data.the reason this is important. This article discusses the selection of starting materials (sms) for the commercial.
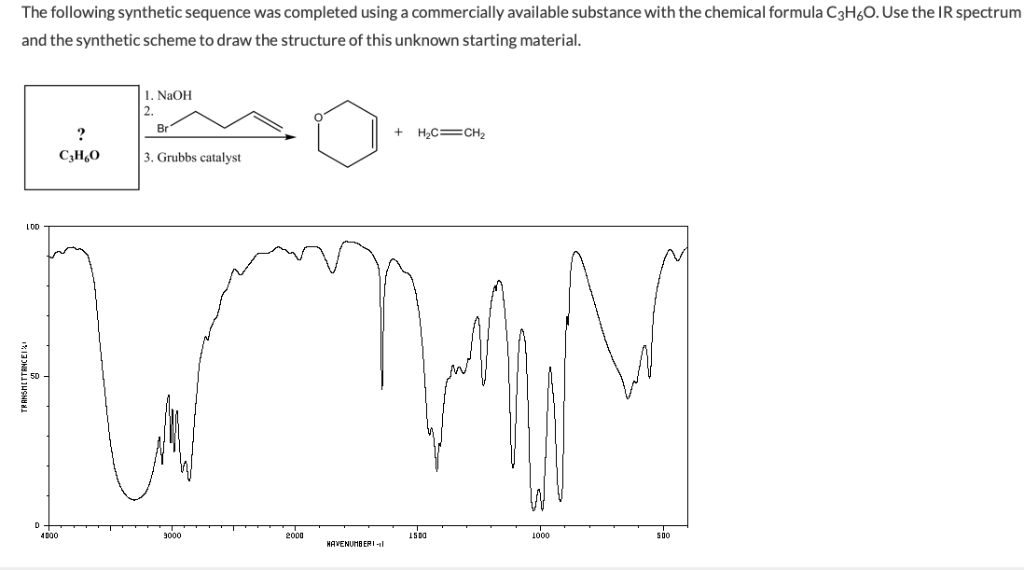
Solved The following synthetic sequence was completed using
This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. Port a regulatory starting material proposal to an agency. A justification for the starting materials should be supplied.
Figure S1. Starting materials All starting materials are listed as
A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for. The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. And the better packages are ones that are supported by data.the reason this is.
Figure 1 from Identification of Commercially Available Oligonucleotide
And the better packages are ones that are supported by data.the reason this is important. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. This article discusses the selection of starting materials (sms) for the commercial manufacture of active pharmaceutical ingredients (apis) and. Port a.
Commercially available starting materials FAQ
The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for. This article discusses the selection of starting materials (sms) for the commercial manufacture of.
Figure 1 from Identification of Commercially Available Oligonucleotide
The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been. And the better packages are ones that are supported by data.the reason this.
This Article Discusses The Selection Of Starting Materials (Sms) For The Commercial Manufacture Of Active Pharmaceutical Ingredients (Apis) And.
The availability of a chemical from multiple suppliers should not be the sole basis for the designation of a chemical as a commercially. A chemical manufactured on a small scale can be suitable as a commercially available starting material provided that the scale is sufficient for. And the better packages are ones that are supported by data.the reason this is important. A justification for the starting materials should be supplied − starting materials can only be justified once the criticality of all steps has been.