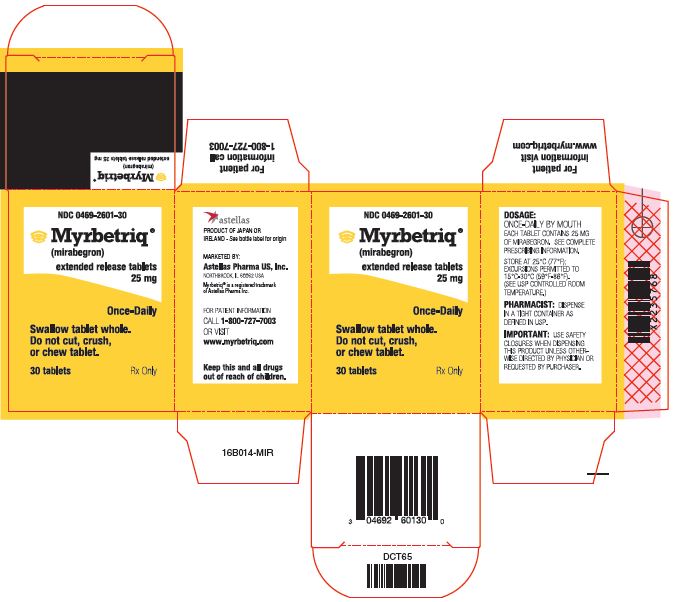
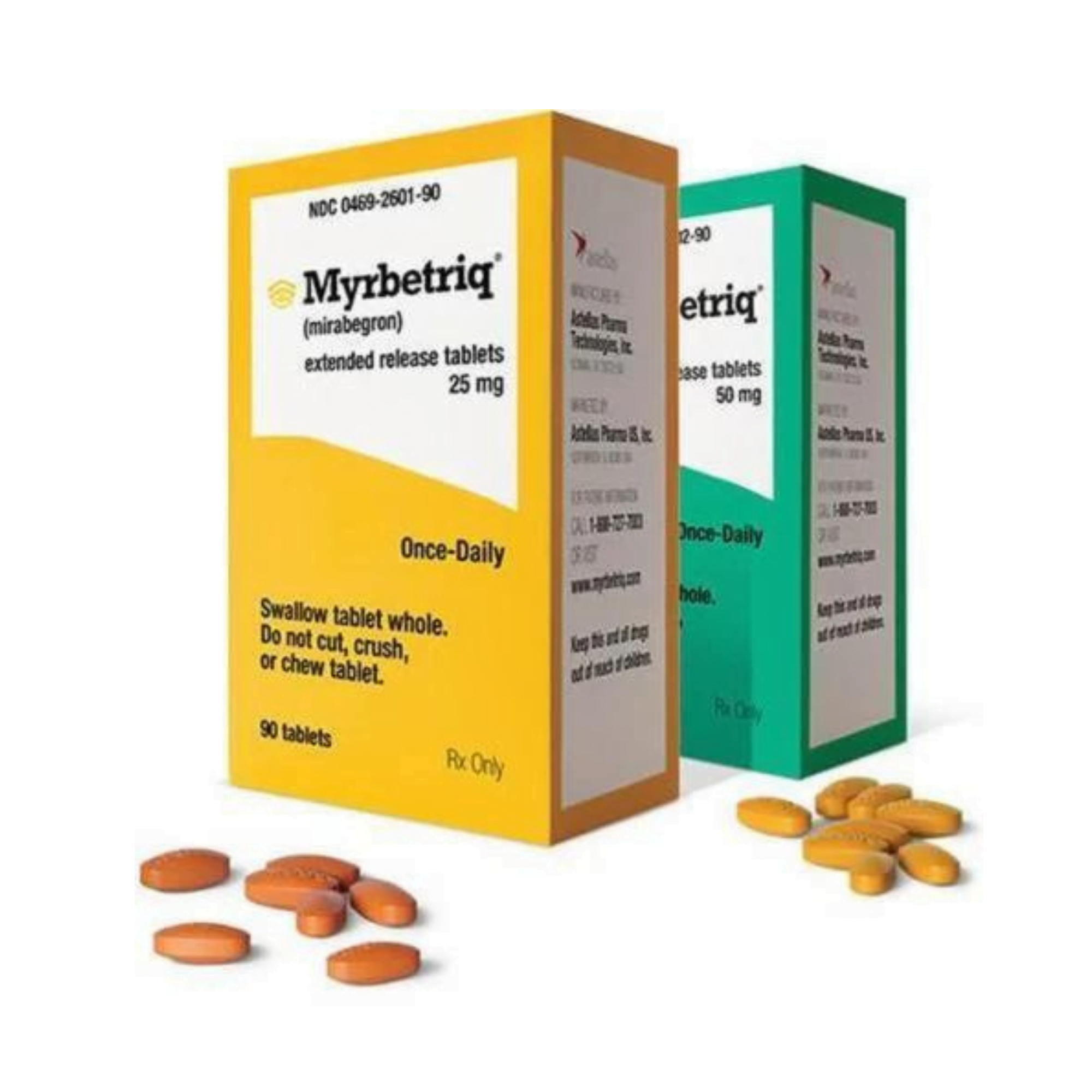
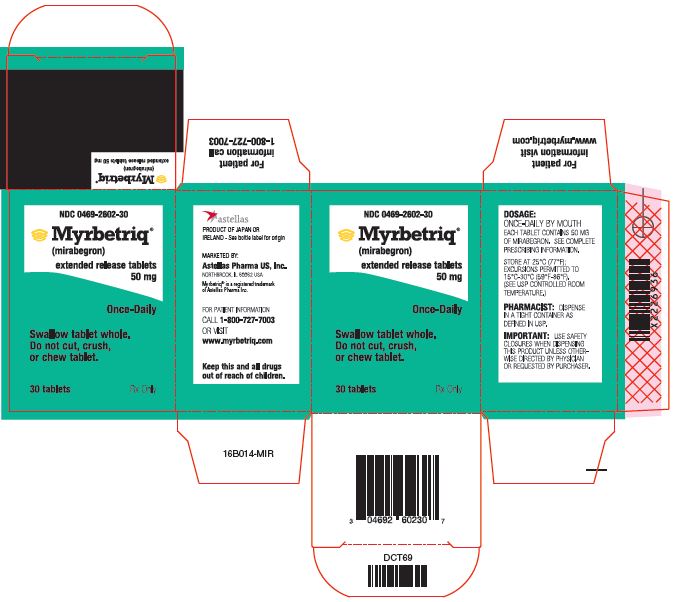
Myrbetriq Generic Availability - Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
The generic version of myrbetriq is expected to be available in late 2024, pending fda approval. Myrbetriq (mirabegron) is taken by mouth once a day.
MYRBETRIC 50 MG 30 TABLETAS
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Myrbetriq FDA prescribing information, side effects and uses
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Buy Myrbetriq online from Canada BFH
The generic version of myrbetriq is expected to be available in late 2024, pending fda approval. Myrbetriq (mirabegron) is taken by mouth once a day.
Buy Myrbetriq from Canada LOWEST PRICE on Mirabegron
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Launch of generic Myrbetriq ER (Mirabegron ER)
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
MyrbetriqER50mg.png
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Buy Myrbetriq for Overactive bladder or Urinary Incontinence Online
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Myrbetriq Package Insert
Myrbetriq (mirabegron) is taken by mouth once a day. The generic version of myrbetriq is expected to be available in late 2024, pending fda approval.
Myrbetriq FDA prescribing information, side effects and uses
The generic version of myrbetriq is expected to be available in late 2024, pending fda approval. Myrbetriq (mirabegron) is taken by mouth once a day.
The Generic Version Of Myrbetriq Is Expected To Be Available In Late 2024, Pending Fda Approval.
Myrbetriq (mirabegron) is taken by mouth once a day.