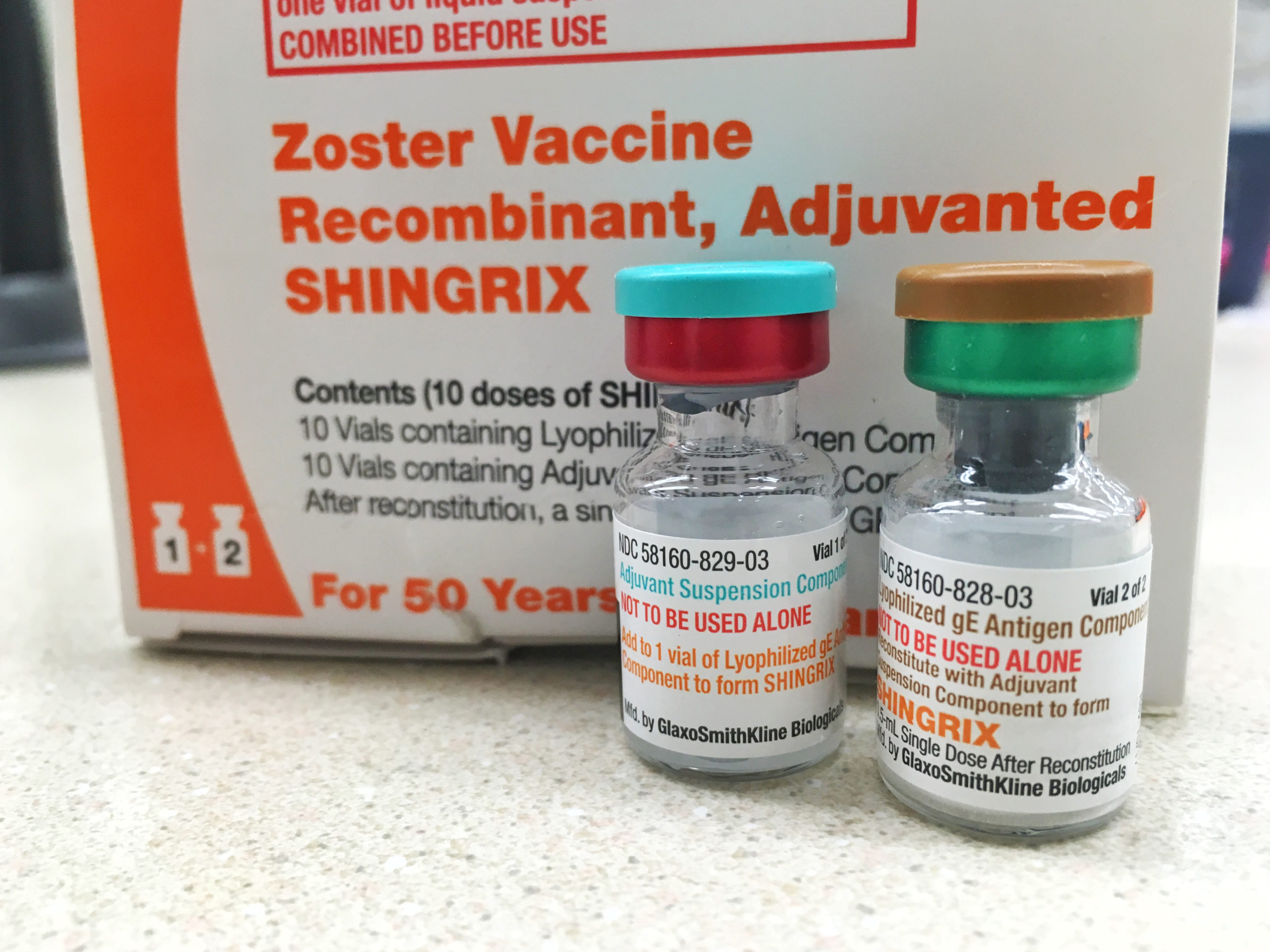
When Did Shingrix Become Available - We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. You are hereby authorized to introduce or deliver for.
You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years.
Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date.
Shingrix, The Shingles Vaccine, Could Reduce Your Risk Of Dementia
Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention.
(Approval lapsed) SHINGRIX Varicella Zoster Virus
Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention.
The Shingles Vaccine SHINGRIX A Good Choice for Preventing Shingles
We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention.
Shingles Vaccination in Bengaluru Herpes Zoster Vaccine at RxDx
We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention.
SHINGRIX
Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. You are hereby authorized.
Shingrix OK'd for Adults MedPage Today
You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially.
SHINGRIX (Zoster Vaccine Adjuvanted) Suspension 50 mcg/0
Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. You are hereby authorized to introduce or deliver for. We have approved your bla for zoster vaccine.
Shingles vaccine is a must for those over 50 The Washington Post
You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially.
Shingles Vaccine Highly Effective Over 4 Years MedPage Today
You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. We have approved your bla for zoster vaccine.
Shingrix Woman's Clinic PA
Studies indicate that immunity from shingrix remains robust for several years after full vaccination—potentially lasting up to ten years. We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. You are hereby authorized to introduce or deliver for. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention.
Studies Indicate That Immunity From Shingrix Remains Robust For Several Years After Full Vaccination—Potentially Lasting Up To Ten Years.
We have approved your bla for zoster vaccine recombinant, adjuvanted effective this date. Shingrix was approved for medical use in the european union in march 2018, with an indication for the prevention of herpes zoster (hz) and. You are hereby authorized to introduce or deliver for.