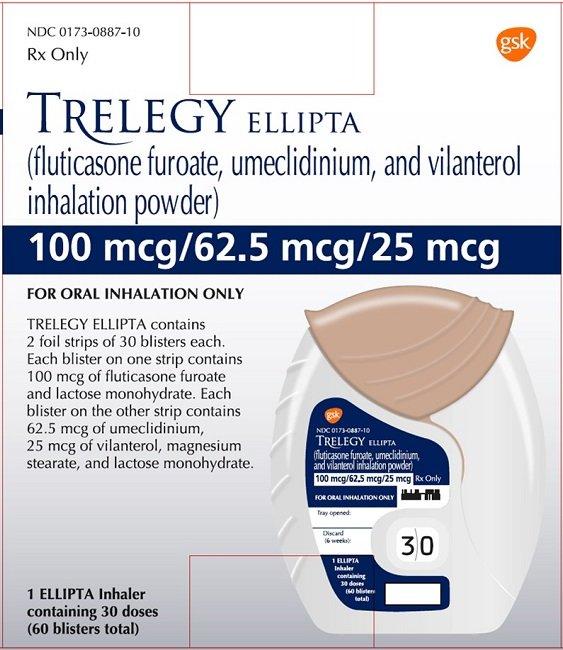
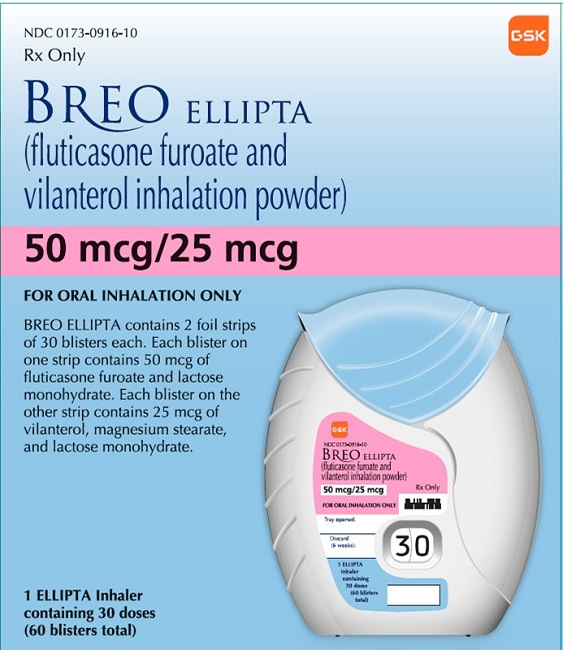
When Will Generic Breo Be Available - The generic version of breo ellipta may potentially be available after the expiration of its last patent. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. This is the exact same active. To be covered and can be filled for the patients. Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. When will breo ellipta generic be available? By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029.
This is the exact same active. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. To be covered and can be filled for the patients. When will breo ellipta generic be available? Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. The generic version of breo ellipta may potentially be available after the expiration of its last patent.
By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. To be covered and can be filled for the patients. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. When will breo ellipta generic be available? This is the exact same active. Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. The generic version of breo ellipta may potentially be available after the expiration of its last patent.
Breo Ellipta 200mcg packaging
This is the exact same active. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. The generic version of breo ellipta may potentially be available after the expiration of its last patent. When will breo ellipta generic be available? Ric breo ellipta (fluticasone/vilanterol) is not covered or may.
Breo Ellipta Label
By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. The generic version of breo ellipta may potentially be available after the expiration of its last patent. This is the exact same active. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. To be.
Breo Ellipta
To be covered and can be filled for the patients. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. This is the exact same active. Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. When will breo ellipta generic be available?
BREO ELLIPTA Dosage & Rx Info Uses, Side Effects The Clinical Advisor
Breo ellipta doesn’t have a generic form, but an authorized generic version is available. Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. The generic version of breo ellipta may potentially be available after the expiration of its last patent. When will breo ellipta generic be available? This is the exact same active.
DailyMed BREO ELLIPTA fluticasone furoate and vilanterol trifenatate
Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. When will breo ellipta generic be available? Breo ellipta doesn’t have a generic form, but an authorized generic version is available. This is the exact same active. To be covered and can be filled for the patients.
Generic Release Of Breo Ellipta Inhaler Is Now Available
This is the exact same active. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. Breo ellipta doesn’t have a generic form, but an authorized generic version is available. The generic version of breo ellipta may potentially be available after the expiration of its last patent. To be.
Breo Ellipta MedEd Center
Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. To be covered and can be filled for the patients. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. When will breo ellipta generic be available? The generic version of breo ellipta may potentially be available.
AmericanPharmaWholesale BREOELLIPTA
When will breo ellipta generic be available? By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. The generic version of breo ellipta may potentially be available after the expiration of its last patent. Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. Breo ellipta doesn’t.
Respiratory Therapy Cave Get Free Breo for one year
By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. To be covered and can be filled for the patients. This is the exact same active. The generic version of breo ellipta may potentially be available after the expiration of its last patent. When will breo ellipta generic be.
Breo Ellipta (Vilanterol Trifenatate/Fluticasone Furoate)(Product Image
When will breo ellipta generic be available? Ric breo ellipta (fluticasone/vilanterol) is not covered or may require prior authoriza. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. To be covered and can be filled for the patients. This is the exact same active.
To Be Covered And Can Be Filled For The Patients.
When will breo ellipta generic be available? By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be august 26, 2029. This is the exact same active. Breo ellipta doesn’t have a generic form, but an authorized generic version is available.
Ric Breo Ellipta (Fluticasone/Vilanterol) Is Not Covered Or May Require Prior Authoriza.
The generic version of breo ellipta may potentially be available after the expiration of its last patent.