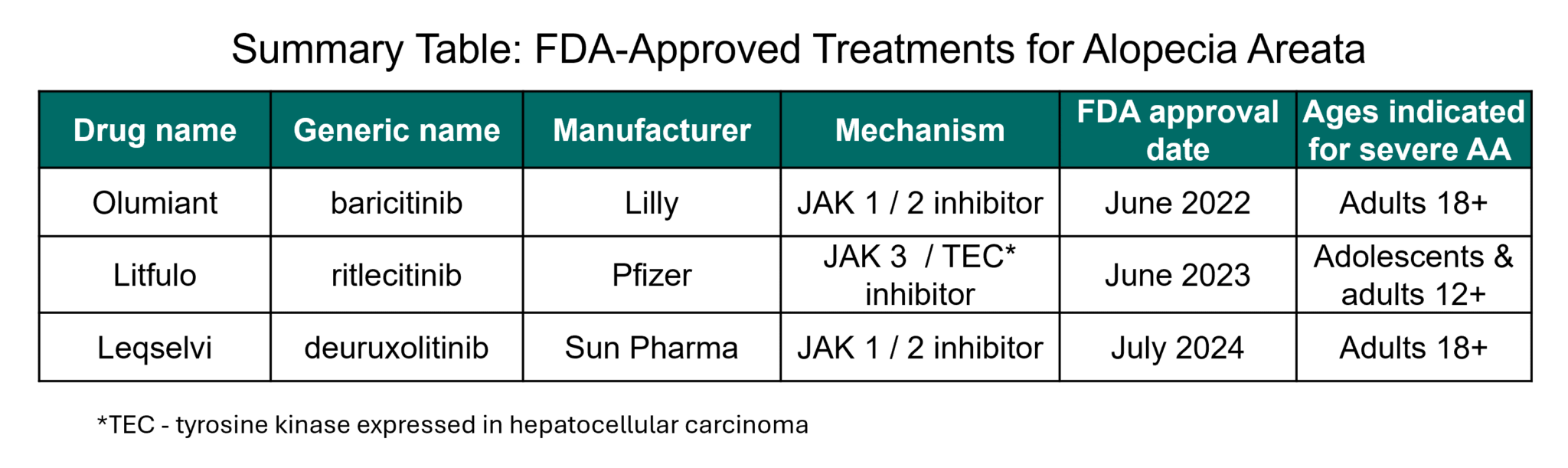
When Will Jak Inhibitors Be Available For Alopecia - Jak inhibitors have changed that; Here, we briefly review the history of and rationale for. Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss.
Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Jak inhibitors have changed that; Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Here, we briefly review the history of and rationale for.
Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Jak inhibitors have changed that; In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Here, we briefly review the history of and rationale for.
FDAApproved JAK Inhibitors National Alopecia Areata Foundation NAAF
Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Here, we briefly review the history of and rationale for. Jak inhibitors have changed that; Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. In july 2024,.
Continuing Medical Education 3 Things You Should Know About Managing
Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Jak inhibitors have changed that; Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases.
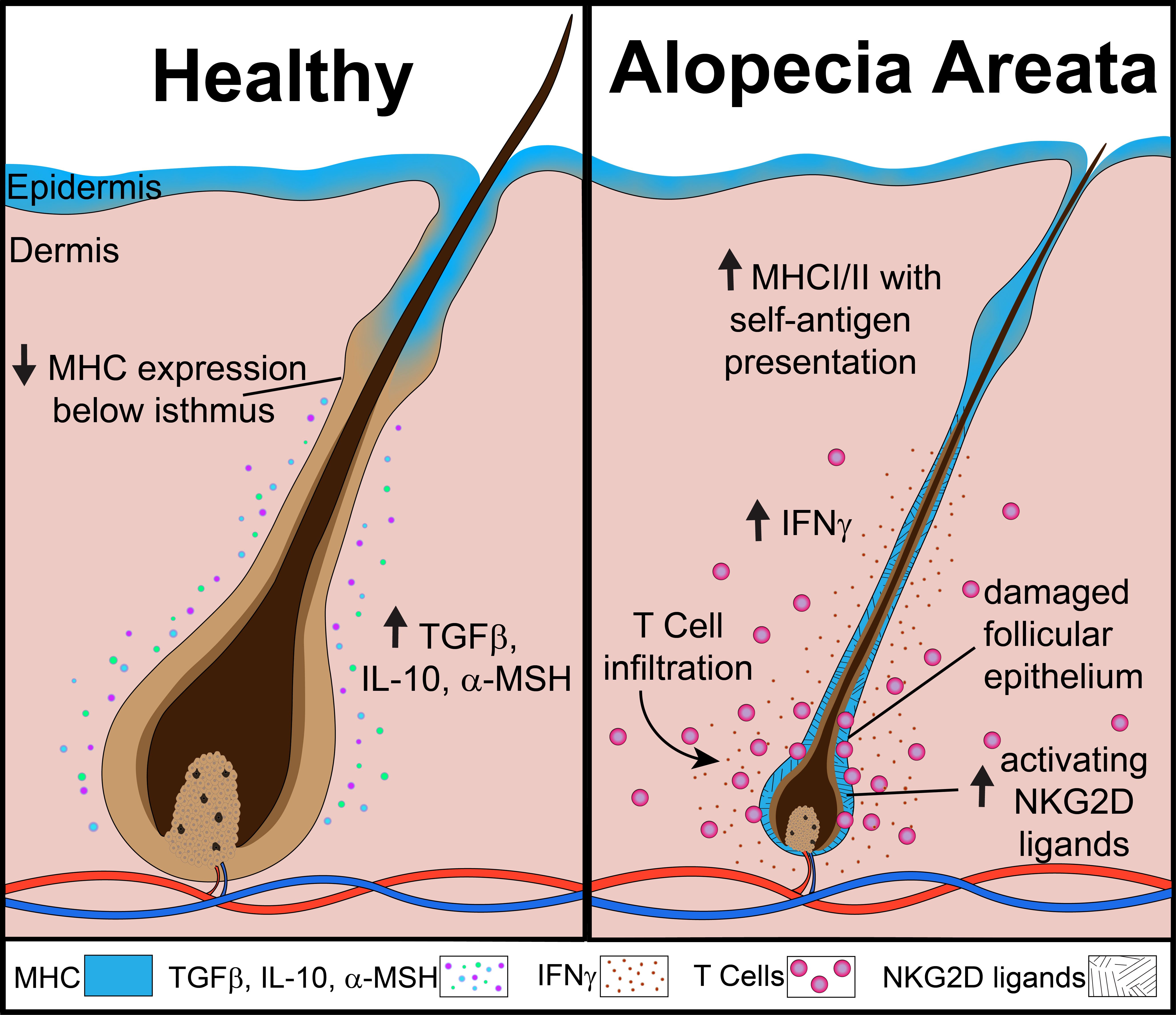
Frontiers An overview of JAK/STAT pathways and JAK inhibition in
Jak inhibitors have changed that; Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Here, we briefly review the history of and rationale for. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. In july 2024,.
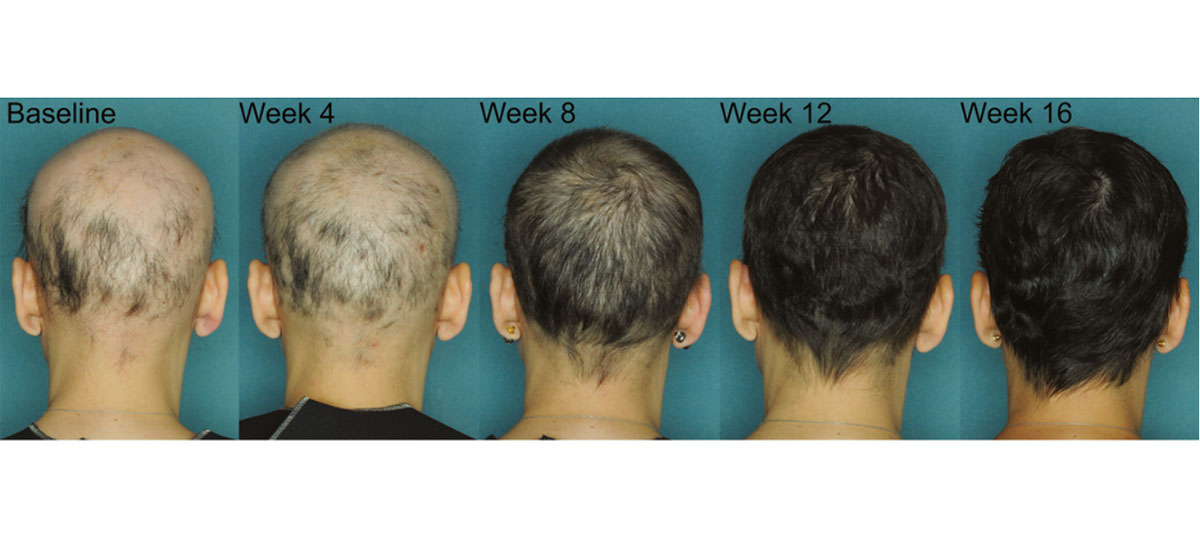
Review Approving, Using JAK Inhibitors for Better Alopecia Areata
Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Here, we briefly review the history of and rationale for. Jak inhibitors have changed that; Currently,.
New Study Confirms JAK3 Effective in Alopecia Areata
Here, we briefly review the history of and rationale for. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Currently, jak inhibitors are only approved.
Understanding JAK Inhibitors Australia Alopecia Areata Foundation
Here, we briefly review the history of and rationale for. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Leqselvi (deuruxolitinib) 8 mg tablets is an oral.
Available Treatments National Alopecia Areata Foundation NAAF
Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Jak inhibitors have changed that; Currently, jak inhibitors are only approved for people who have severe.
Third JAK Inhibitor for Alopecia Areata Wins FDA Approval
Jak inhibitors have changed that; In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Here, we briefly review the history of and rationale for. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Leqselvi (deuruxolitinib) 8.
Continuing Medical Education Resources National Alopecia Areata
Here, we briefly review the history of and rationale for. Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Jak inhibitors have changed that; Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. In july 2024,.
First Oral JAK Inhibitor Approved for Severe Alopecia Areata in Teens
In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for.
Here, We Briefly Review The History Of And Rationale For.
Leqselvi (deuruxolitinib) 8 mg tablets is an oral selective inhibitor of janus kinases jak1 and jak2 approved for the treatment of adult. Currently, jak inhibitors are only approved for people who have severe alopecia areata—those with 50 percent or more hair loss. Jak inhibitors have changed that; In july 2024, the fda approved a third jak inhibitor, deuruxolitinib (leqselvi®), for the treatment of alopecia areata in adults 18 years.