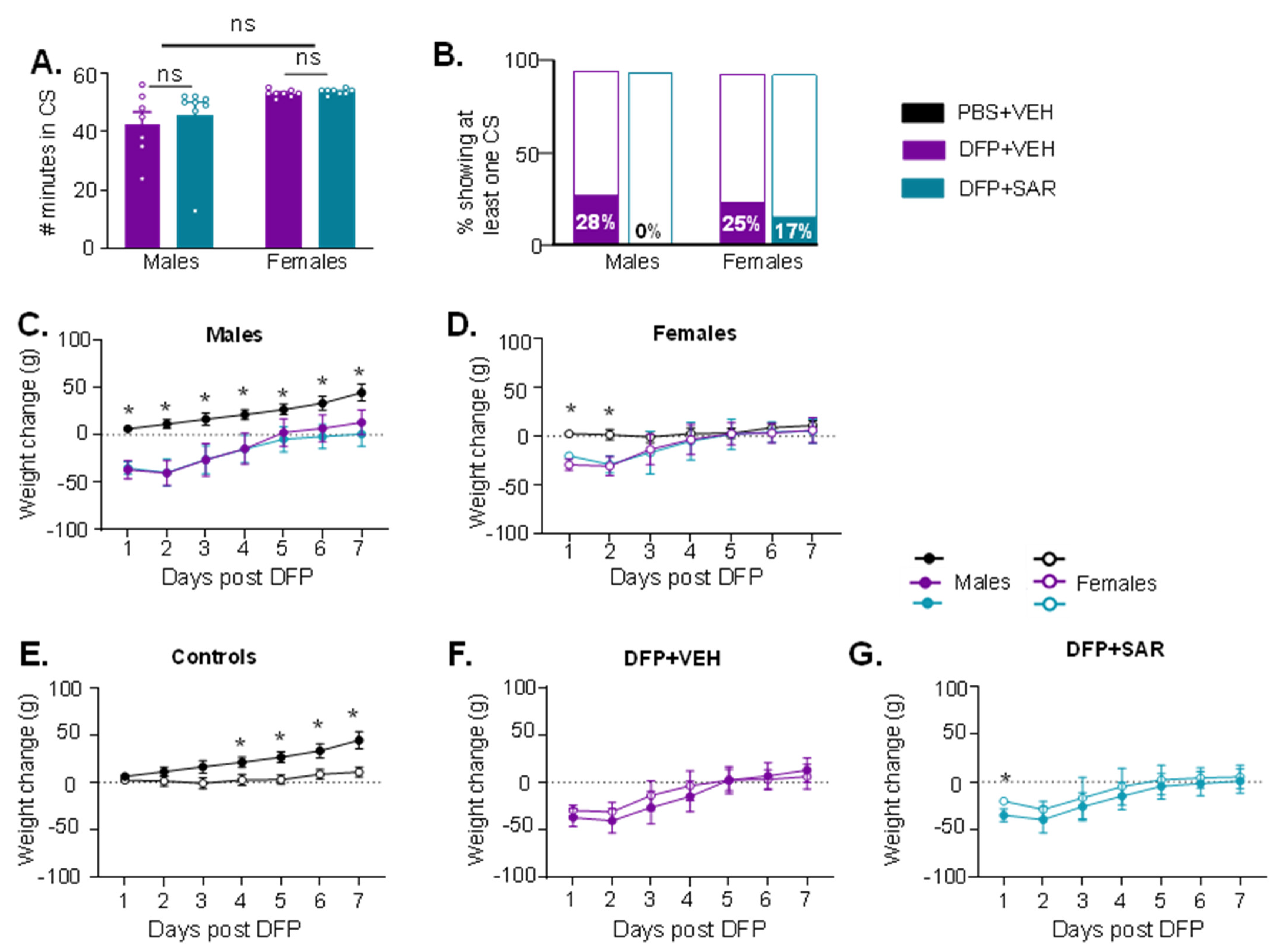
When Will Saracatinib Be Available For Ipf - However, i cannot see any preliminary reports, and may. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. When i read about sarcatinib, i found that the trial is call, stop ipf. The trial’s primary outcome is safety;.
However, i cannot see any preliminary reports, and may. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. When i read about sarcatinib, i found that the trial is call, stop ipf. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. The trial’s primary outcome is safety;. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new.
The trial’s primary outcome is safety;. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. When i read about sarcatinib, i found that the trial is call, stop ipf. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. However, i cannot see any preliminary reports, and may.
A schematic model of the antifibrotic mechanism of Saracatinib
In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The trial’s primary outcome is safety;. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late.
Saracatinib enhances the efficacy of ispinesib in murine and human GBM
Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. However, i cannot see any preliminary reports, and may. When i read about sarcatinib, i found that the trial is call, stop ipf. The trial’s primary outcome.
Saracatinib for Idiopathic Pulmonary Fibrosis Clinical Trial 2025 Power
When i read about sarcatinib, i found that the trial is call, stop ipf. However, i cannot see any preliminary reports, and may. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university.
Antioxidants Free FullText Saracatinib, a Src Tyrosine Kinase
The trial’s primary outcome is safety;. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. In the trial, which began in 2020, ipf patients receive either saracatinib or.
SRCinhibitor saracatinib abrogates bronchosphere formation af
The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. When i read.
Saracatinib is a selective inhibitor of BMP versus TGFβ type I
When i read about sarcatinib, i found that the trial is call, stop ipf. The trial’s primary outcome is safety;. However, i cannot see any preliminary reports, and may. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a.
A schematic model of the antifibrotic mechanism of Saracatinib
When i read about sarcatinib, i found that the trial is call, stop ipf. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer.
HNSCC cells exhibit differential response to saracatinib. a The effect
When i read about sarcatinib, i found that the trial is call, stop ipf. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib,.
SRCinhibitor saracatinib attenuates fibrosis in the humanized mouse
However, i cannot see any preliminary reports, and may. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The trial’s primary outcome is safety;. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The us food and drug administration (fda) has granted orphan drug designation (odd) for.
Saracatinib suppressed Fyn/FAK/NWASP pathway. Western blot analysis on
However, i cannot see any preliminary reports, and may. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. The stop ipf trial is expected to be completed within a year from now, which suggests that results.
Recent Investigations From Tulane University Reveal That Saracatinib, Initially Developed For Oncology, May Offer A New.
The trial’s primary outcome is safety;. However, i cannot see any preliminary reports, and may. When i read about sarcatinib, i found that the trial is call, stop ipf. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo.
The Stop Ipf Trial Is Expected To Be Completed Within A Year From Now, Which Suggests That Results May Be Available By Late 2025 [7].
The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential.


:%0A%0ASaracatinib for Idiopathic Pulmonary Fibrosis.png?md=1)