Why Is Ketoprofen No Longer Available - Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. A company may have decided to no longer produce ketoprofen. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Its trometamol salt is said to be particularly. This decision could be due to any number of reasons, including the. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain.
Its trometamol salt is said to be particularly. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. This decision could be due to any number of reasons, including the. A company may have decided to no longer produce ketoprofen.
A company may have decided to no longer produce ketoprofen. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. Its trometamol salt is said to be particularly. This decision could be due to any number of reasons, including the.
Ketoprofen Information, Side Effects, Warnings and Recalls
This decision could be due to any number of reasons, including the. Its trometamol salt is said to be particularly. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. A company may have decided to no longer produce ketoprofen. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed.
Ketoprofen Tablet Ketoprofen And Thiocolchicoside Tablets Latest
The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. A company may have decided to no longer produce ketoprofen. This decision could be due to any number of reasons, including the. Its trometamol salt.
Ketoprofen Information, Side Effects, Warnings and Recalls
The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. This decision could be due to any number of reasons, including the. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. Its trometamol salt is said to be particularly. A company may have decided.
Ketoprofen 100 mg 10 Tablet Novell Manfaat, Kandungan, Dosis, dan
The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. This decision could be due to any number of reasons, including the. A company may have decided to no longer produce ketoprofen. Its trometamol salt is said to be particularly. Teva responded right away, saying ketoprofen is currently unavailable and there is no.
Ketoprofen Tablet Ketoprofen And Thiocolchicoside Tablets Latest
The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. Its trometamol salt is said to be particularly. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and.
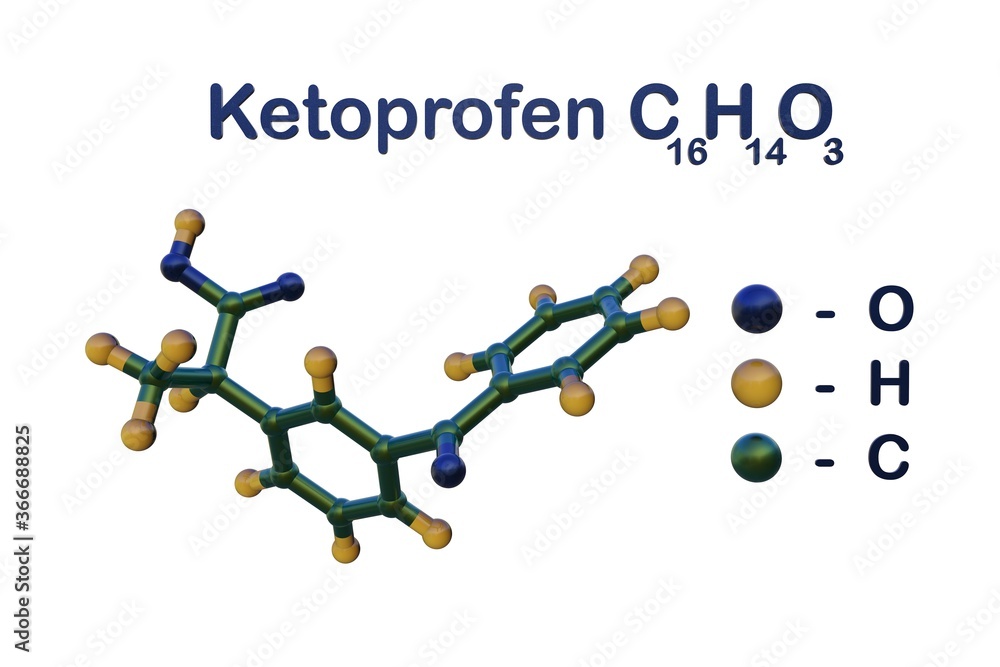
Structural chemical formula and molecular model of ketoprofen, the
This decision could be due to any number of reasons, including the. Its trometamol salt is said to be particularly. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain. Teva responded right away, saying.
BUY Ketoprofen ER 200 Ketoprofen USP 200 mg by Mylan Pharmaceuticals
A company may have decided to no longer produce ketoprofen. Its trometamol salt is said to be particularly. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. This decision could be due to any.
Cairo, Egypt, September 13 2023 Ketofan Ketoprofen 50mg capsules, an
Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. Its trometamol salt is said to be particularly. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain. A company may have decided to no longer produce ketoprofen. The food and drug administration (fda).
Jual KETOPROFEN 50MG 1 STRIP 10 TABLET Shopee Indonesia
A company may have decided to no longer produce ketoprofen. Its trometamol salt is said to be particularly. Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were. This decision could be due to any.
Ketoprofen 100 mg 10 Tablet Hexpharm Manfaat, Kandungan, Dosis, dan
Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. Ketoprofen (nexcede, orudis, oruvail, actron brands have been discontinued) is a nsaid prescribed to treat inflammation and pain. Its trometamol salt is said to be particularly. A company may have decided to no longer produce ketoprofen. This decision could be due to.
Its Trometamol Salt Is Said To Be Particularly.
Teva responded right away, saying ketoprofen is currently unavailable and there is no release date at this time. This decision could be due to any number of reasons, including the. A company may have decided to no longer produce ketoprofen. The food and drug administration (fda) has determined that orudis kt (ketoprofen) tablets, 12.5 milligrams (mg), were.