When Will Troriluzole Be Available - Food and drug administration (fda) for troriluzole for the. No new concerns raised, advisory meeting. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; A new drug application (nda) has been submitted to the u.s.
Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; Food and drug administration (fda) for troriluzole for the. No new concerns raised, advisory meeting. A new drug application (nda) has been submitted to the u.s.
No new concerns raised, advisory meeting. Food and drug administration (fda) for troriluzole for the. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; A new drug application (nda) has been submitted to the u.s.
GRAPHIC
A new drug application (nda) has been submitted to the u.s. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; No new concerns raised, advisory meeting. Food and drug administration (fda) for troriluzole for the.
Biohaven Achieves Positive Topline Results in Pivotal Study of
A new drug application (nda) has been submitted to the u.s. No new concerns raised, advisory meeting. Food and drug administration (fda) for troriluzole for the. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;
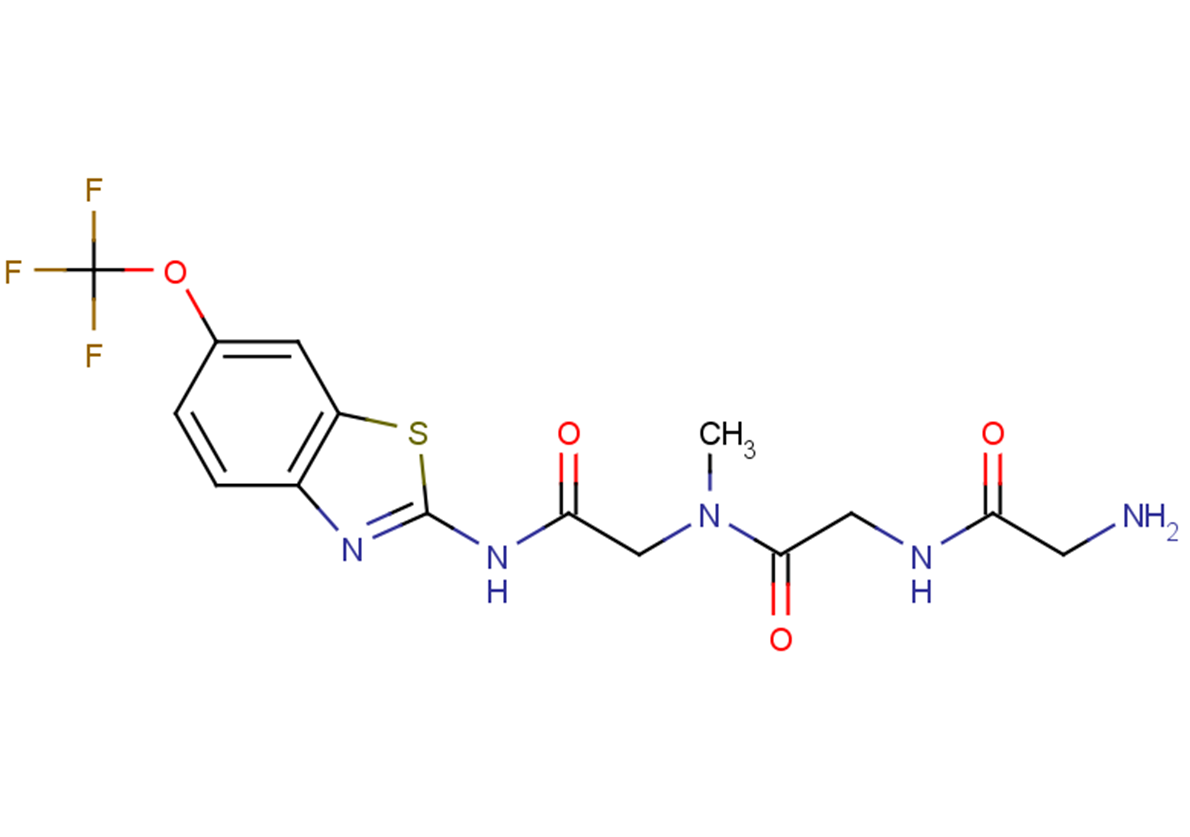
Troriluzole (BHV4157) Glutamate Modulator MedChemExpress
Food and drug administration (fda) for troriluzole for the. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; No new concerns raised, advisory meeting. A new drug application (nda) has been submitted to the u.s.
Troriluzole Application in Therapy and Current Clinical Research
Food and drug administration (fda) for troriluzole for the. No new concerns raised, advisory meeting. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; A new drug application (nda) has been submitted to the u.s.
Troriluzole and riluzole concentrations (ng/mL) in human EDTA K2
No new concerns raised, advisory meeting. A new drug application (nda) has been submitted to the u.s. Food and drug administration (fda) for troriluzole for the. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;
Troriluzole Rational Drug Discovery to Optimize Therapy Improved
No new concerns raised, advisory meeting. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; A new drug application (nda) has been submitted to the u.s. Food and drug administration (fda) for troriluzole for the.
Troriluzole (BHV4157) for Disorder Clinical Trial
Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data; A new drug application (nda) has been submitted to the u.s. No new concerns raised, advisory meeting. Food and drug administration (fda) for troriluzole for the.
(PDF) A phase Ib doseescalation study of troriluzole (BHV4157), an
No new concerns raised, advisory meeting. A new drug application (nda) has been submitted to the u.s. Food and drug administration (fda) for troriluzole for the. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;
Troriluzole Inhibitor TargetMol
No new concerns raised, advisory meeting. Food and drug administration (fda) for troriluzole for the. A new drug application (nda) has been submitted to the u.s. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;
Troriluzole Rational Drug Discovery to Optimize Therapy Improved
Food and drug administration (fda) for troriluzole for the. A new drug application (nda) has been submitted to the u.s. No new concerns raised, advisory meeting. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;
Food And Drug Administration (Fda) For Troriluzole For The.
A new drug application (nda) has been submitted to the u.s. No new concerns raised, advisory meeting. Fda extends pdufa date for troriluzole nda in sca to q4 2025 to review new data;






:%0A%0ATroriluzole (BHV-4157) for Obsessive-Compulsive Disorder.png?md=1)