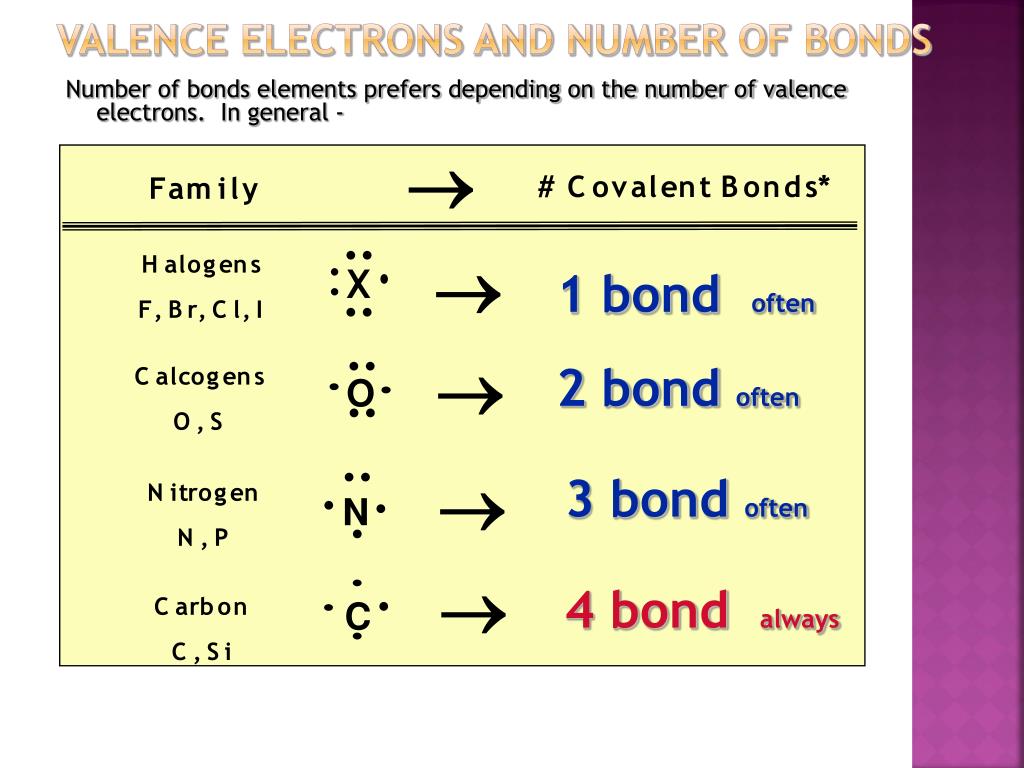
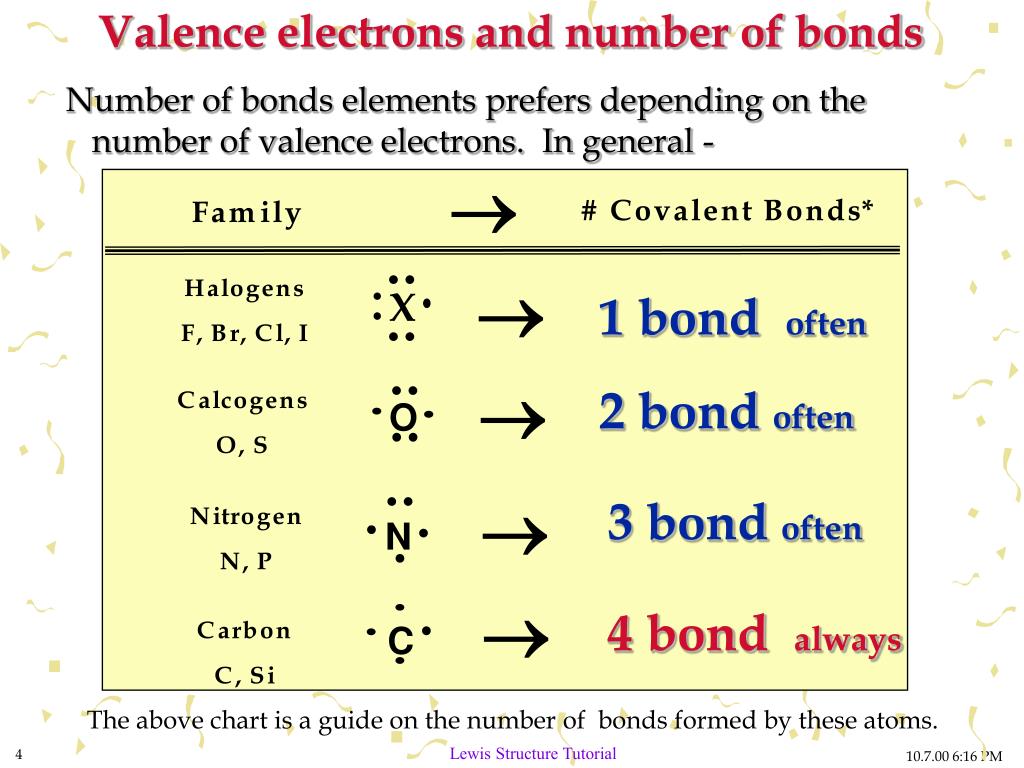
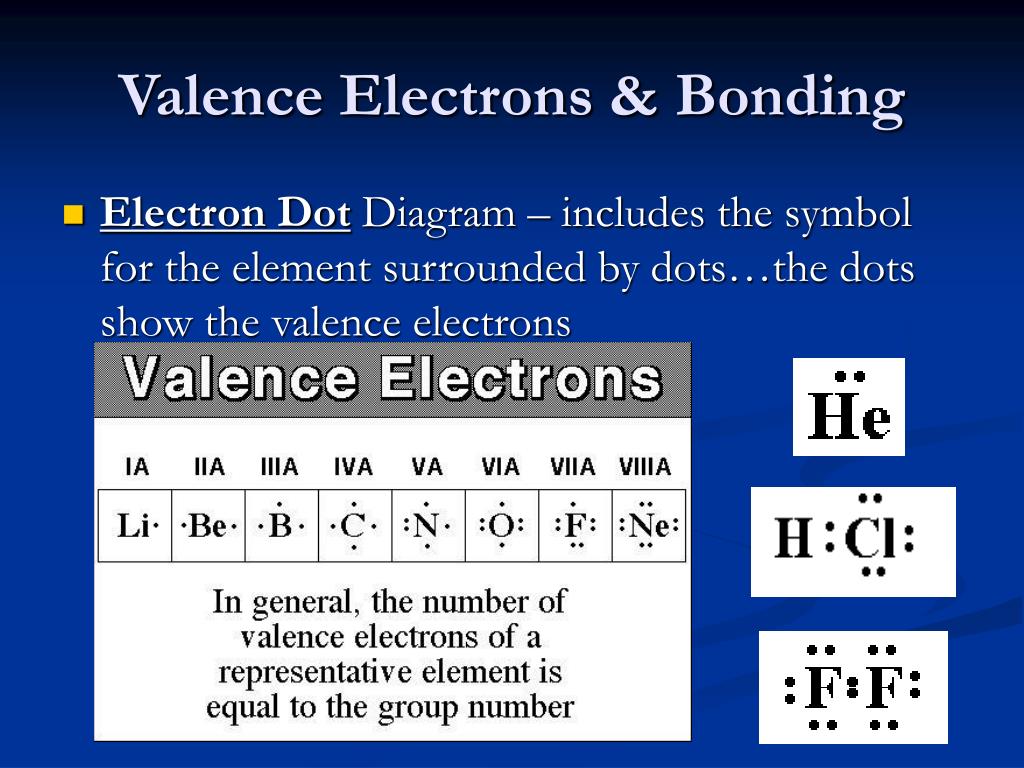
Which Element Has The Fewest Valence Electrons Available For Bonding - Nitrogen (n) has 5 valence electrons. A) iodine b) aluminum c) carbon d) nitrogen Which element has the fewest valence electrons available for bonding? Aluminum (al) has 3 valence electrons. The total number of electrons present in the outer. Iodine (i) has 7 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Carbon (c) has 4 valence electrons. This is typical for elements in group. Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,.
A) iodine b) aluminum c) carbon d) nitrogen The total number of electrons present in the outer. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. Which element has the fewest valence electrons available for bonding? Aluminum (al) has 3 valence electrons. Nitrogen (n) has 5 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Iodine (i) has 7 valence electrons. Carbon (c) has 4 valence electrons.
Sodium (na) with atomic number 11 has the least number of valence electrons. Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Aluminum (al) has 3 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Nitrogen (n) has 5 valence electrons. Carbon (c) has 4 valence electrons. A) iodine b) aluminum c) carbon d) nitrogen The total number of electrons present in the outer. This is typical for elements in group. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence.
PPT Bonding PowerPoint Presentation, free download ID6281432
A) iodine b) aluminum c) carbon d) nitrogen This is typical for elements in group. Carbon (c) has 4 valence electrons. Which element has the fewest valence electrons available for bonding? Iodine (i) has 7 valence electrons.
[Solved] QUESTION 43 Which element has the fewest valence electrons? H
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Which element has the fewest valence electrons available for bonding? Carbon (c) has 4 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Sodium (na) with atomic number 11 has the least number.
Chemistry Shearer Standard 1 ppt download
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Iodine (i) has 7 valence electrons. Which element has the fewest valence electrons available for bonding? Carbon (c) has 4 valence electrons. Aluminum (al) has 3 valence electrons.
PPT Chemical Bonding and Molecular Structure PowerPoint Presentation
This is typical for elements in group. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. Nitrogen (n) has 5 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Which element has the fewest valence electrons available for bonding?
PPT Drawing Lewis Structures A Tutorial on Writing Lewis Dot
Iodine (i) has 7 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. A) iodine b) aluminum c) carbon d) nitrogen Which element has the fewest valence electrons available for bonding?
Lesson 3 Bonding & the Periodic Table Diagram Quizlet
Carbon (c) has 4 valence electrons. Nitrogen (n) has 5 valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. This is typical for elements in group.
Elements and Chemical Bonds
Iodine (i) has 7 valence electrons. This is typical for elements in group. Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Aluminum (al) has 3 valence electrons. The total number of electrons present in the outer.
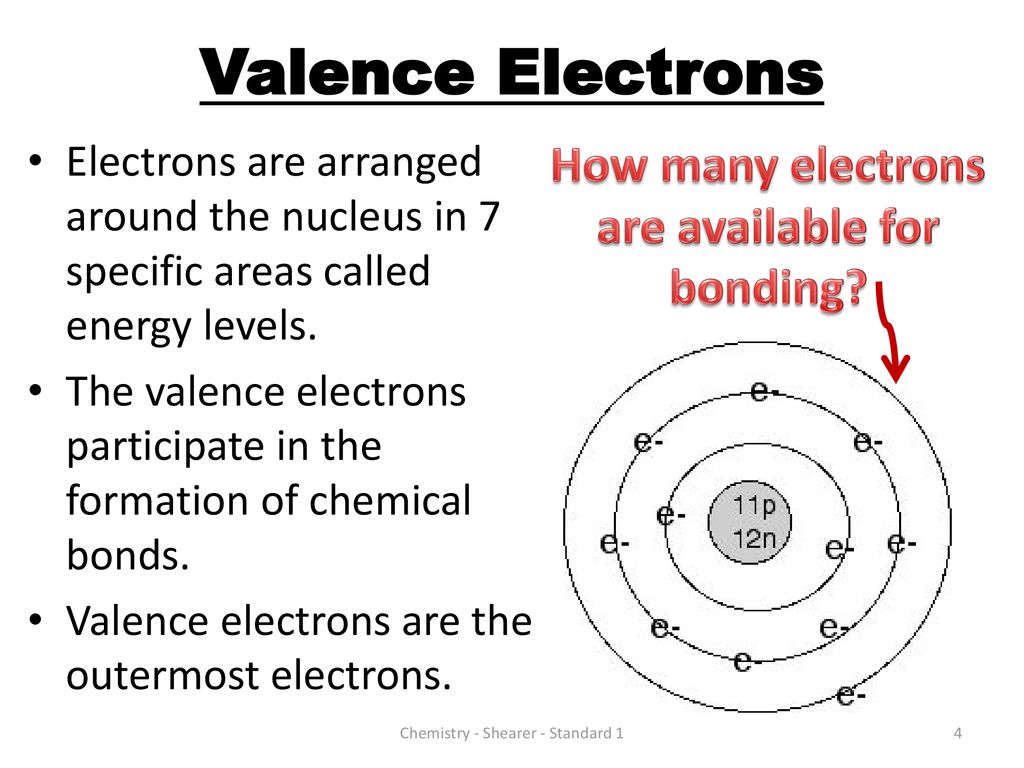
Valence Electrons ELECTRONS AVAILABLE FOR BONDING CA Standards
Sodium (na) with atomic number 11 has the least number of valence electrons. A) iodine b) aluminum c) carbon d) nitrogen Nitrogen (n) has 5 valence electrons. Carbon (c) has 4 valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence.
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation, free
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. A) iodine b) aluminum c) carbon d) nitrogen The total number of electrons present in the outer. Aluminum (al) has 3 valence electrons. Iodine (i) has 7 valence electrons.
PPT Intro to Bonding Part 2 Covalent Compounds (Type 3 Binary
Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Nitrogen (n) has 5 valence electrons. Which element has the fewest valence electrons available for bonding? The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. The total number of electrons present in the outer.
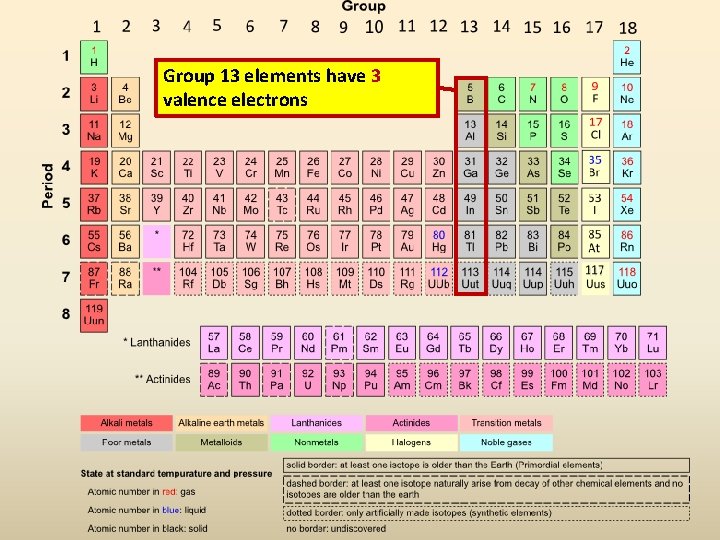
Aluminum (Al) Has 3 Valence Electrons.
Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Which element has the fewest valence electrons available for bonding? Nitrogen (n) has 5 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons.
The Element With The Fewest Valence Electrons Available For Bonding Is Lithium (Li), Located In Group 1, Which Has 1 Valence.
The total number of electrons present in the outer. This is typical for elements in group. A) iodine b) aluminum c) carbon d) nitrogen Iodine (i) has 7 valence electrons.
Carbon (C) Has 4 Valence Electrons.
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,.